When you’re tasked with protecting your controlled environment, encapsulating the operator to effectively protect the process and the product is critical.
Clean manufactured garments are an essential tool to meet the strict cleanroom requirements of the pharmaceutical, biotech, life sciences, medical device, nanotechnology, microelectronics and industries utilizing controlled environments. As you look for the right garment for your cleanroom/controlled environment, there are many quality factors to consider, including:
- Cleanroom designation, application and classification
- Manufacturing and sterility data support
- Product availability and accessibility
- Product features and benefits
- Level of customer support
Reviewing these categories in detail with your manufacturer can help you determine if their cleanroom products are appropriate to meet your unique needs.
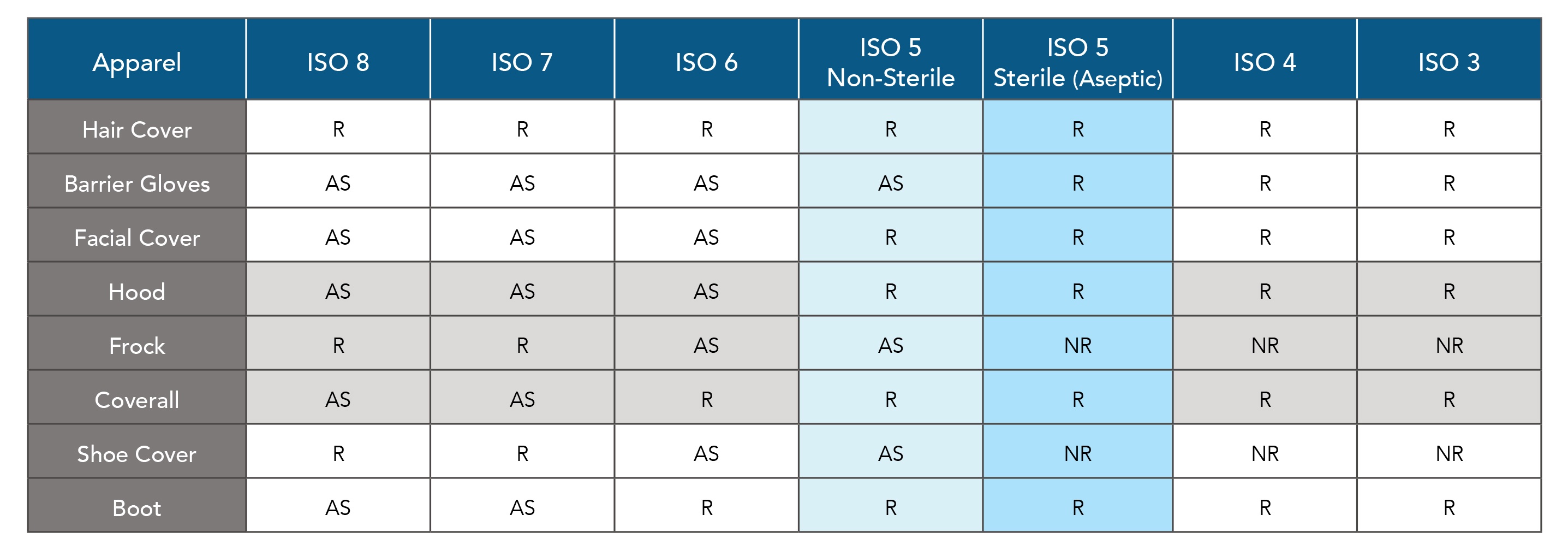
Understanding Your Cleanroom Classifications
Sterilization Level
Sterilization is a process that is used to destroy viable forms of microbial life including, but not limited to: bacteria, viruses, mold, yeast, and algae to an acceptable sterility assurance level (SAL).
For example, Lakeland CleanMax garments with a SAL of 10-⁶ means that there is a one-in-a-million chance that a microorganism will remain after the sterilization process is complete.
Personal Protective Equipment Categories
The U.S. Occupational Safety and Health Administration (OSHA) ensures that employers supply employees with the appropriate protection from environmental hazards. Personal Protective Equipment (PPE) is required to protect the full body, head, eyes and breathing from exposure to chemical penetration. The right level of protection depends on the level of exposure in the working environment.
Lakeland CleanMax™ offers CE 0321 PPE Cat 3 Type 5 & 6 certification, which means:
- Type 5: Protection against hazardous dry particles
- Type 6: Protection against hazardous liquid spray chemicals
ISO Classifications
Cleanrooms are classified by the International Standards Organization (ISO) according to the number and size of particles permitted per volume of air.
- Lakeland CleanMax™ is ready for use in ISO Class 4 – 8 Cleanrooms
IEST-RP-CC003 Category I Particle Cleanliness
Institute of Environmental Science and Technology (IEST) recently published a revision to “Garment Considerations for Cleanrooms and Other Controlled Environments”. Document IEST-RP-CC003.4 outlines all aspects of cleanroom garment systems including the recommended components and construction of cleanroom garments. It is important to ensure that your garment manufacturer is audited for compliance to IEST-RP-CC003.4.
How is Your Cleanroom Garment Manufactured?
Do you know the manufacturing process of your cleanroom garment? If you don’t, now is the time to ask. There is an important difference between ‘sterile’ and ‘clean and sterile’ garments.
- A ‘clean and sterile’ garment is both sterile and clean manufactured, which means the garment is sewn and constructed in a controlled, clean-manufacturing environment.
- A clean-manufacturing facility ensures that operators are not adding any particulates to garments during the construction process.
Manufacturing and Sterility Data Support

At Lakeland, we make it easy for you to see and access the chain of custody for garment sterility. In fact, it’s as easy as 1-2-3.
- Locate the QR code for you garment (it can be found on the garment tag, the individual package and on the box used for shipping.)
- Scan the QR code to access the Lakeland website database.
- View and print your sterility certificate as needed.
When you choose Lakeland CleanMax™ apparel, we make it easy for you to access and print sterility certification.
Look for the Red Indicator Dot of Sterility
Quickly assess the sterility of your garments with a gamma indicator dot, which acts as a visual indication that the product has been exposed to gamma radiation. Lakeland CleanMax™ Sterile offers individually wrapped garments with an expiration date and a self-adhesive, non toxic indicator sticker that changes from yellow to red upon exposure to a validated dose of gamma irradiation.
Moreover, each case is also equipped with a date of irradiation and a sterility indicator to ensure that upon arrival, your garments meet the necessary sterility requirements for your cleanroom classification.
Quick Selection Guide : Who Should Use CleanMax?
Confidence in your cleanroom starts with understanding how to select the right disposable apparel for your unique needs. Part of the benefit of working with Lakeland is ongoing access to our team of cleanroom industry experts. In just a few minutes, we will work with you to determine the type of garment required for your application and environment, and discuss how we can help you protect your team effectively with clean-manufactured garments.
Applications for CleanMax Cleanroom Apparel
CleanMax Clean Sterile
- Aseptic or Terminally Sterile Cleanroom Environments
- ISO Class 4-8 Cleanroom
- Sterility assurance level of 10-6 SAL per ANSI/AAMI ISO1137:2006 (R2011)
CleanMax Clean Manufactured
- ISO Class 4-8 or Below Non-Aseptic Cleanrooms or Controlled Environments
Why Partner with Lakeland Industries?
Lakeland CleanMax garments provide the comfort, quality and protection you expect, all backed by our 30+ years as a manufacturer of disposable protective apparel.
Availability is Essential
Product availability is critical for your controlled environment. Losing valuable production hours because you lack cleanroom apparel is not an option. Our team at Lakeland and our network of exceptional distributors will help you find the right garment for your application and ensure you get your order when you need it with our inventory management program.
Customer Service is Our Strength
At Lakeland, superior customer service is one of our biggest strengths. With a small, highly trained team dedicated customer satisfaction, we are always ready to assist. Whether it is pairing the right customer with the right distributors, obtaining technical data for audits, or securing sterility certificates, our team at Lakeland will work directly with you to meet your needs. Moreover, we are ready to help you protect your people and your environment if your product or garment needs change over time.
At Lakeland, we are here to support your team and to help you feel secure in your investment to protect your people, your controlled environment and your product.
Your cleanroom classification requires you to meet ISO14644 and IEST recommended practices, but have you thought about the particle count and excursions on your cleanroom garments as a result of the manufacturing process? The primary objective of cleanroom gowning is to eliminate contamination risks. Are you positive your cleanroom garments are not introducing more contaminants?